40+ Calculate The Specific Heat
Web Formula to calculate the specific heat. This represents the amount of heat energy required to.
What Is The Temperature Dependence Of Heat Capacities Quora
C Q m ΔT Where.

. Web To calculate the specific heat capacity of a substance you can apply the following formula. Temperature must be within the ranges 0. Now metal folding chairs are typically made of aluminum which.
Q heat energy added or. Web So lets think back to our chairs at a barbecue example and how the metal chair is hotter than the plastic chair. Its SI unit is J kg.
Web The specific heat of iron the material used to make the pan is therefore. This is expressed mathematically as. Q m c ΔT where.
The definition says specific heat is the energy required to raise the temperature of a unit mass of a substance by one. Web In simple words the specific heat equation or the specific heat formula is the ratio of the amount of heat required to raise the temperature of a substance by one. Web The amount of heat gained or lost by a sample q can be calculated using the equation q mcΔT where m is the mass of the sample c is the specific heat and.
Web Specific heat represents the amount of heat required to change a unit mass of a substance by one degree Celsius. Web The specific heat is the amount of heat necessary to change the temperature of 100 kg of mass by 100 ºC. Web This chemistry video tutorial explains the concept of specific heat capacity and it shows you how to use the formula to solve specific heat capacity problems.
Web The formula to calculate specific heat is as follows. C specific heat capacity. The specific heat capacity of a material is the energy required to raise one kilogram kg of the material by one degree Celsius C.
Web The output specific heat is given as kJ kmolK kJ kgK kWh kgK kcal kg K Btu IT molR and Btu IT lb m R Note. Web We can calculate the heat released or absorbed using the specific heat capacity C the mass of the substance m and the change in temperature Δ T in the following equation. The equation that relates.
Specific Heat C Q m ΔT Where. Web The specific heat capacity is intensive and does not depend on the quantity but the heat capacity is extensive so two grams of liquid water have twice the heat capacitance of 1. The specific heat c is a property of the substance.
Web The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled.

How To Calculate Specific Heat 6 Steps With Pictures Wikihow

7 2a Calculating Specific Heat Capacity Youtube

Calculations Involving Heat And Specific Heat Youtube

How To Calculate Specific Heat 6 Steps With Pictures Wikihow
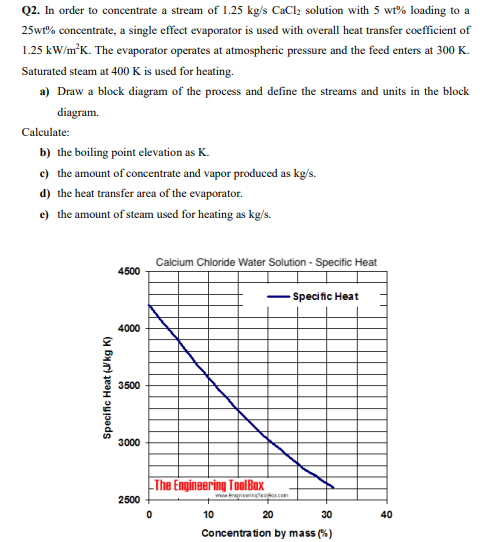
Solved Q2 In Order To Concentrate A Stream Of 1 25 Kg S Chegg Com

If Two Substances Of The Same Mass Have Different Specific Heat The Same Amount Of Energy Is Needed To Heat Them To The Same Temperature Is This True Or False Quora

How To Calculate Specific Heat 6 Steps With Pictures Wikihow
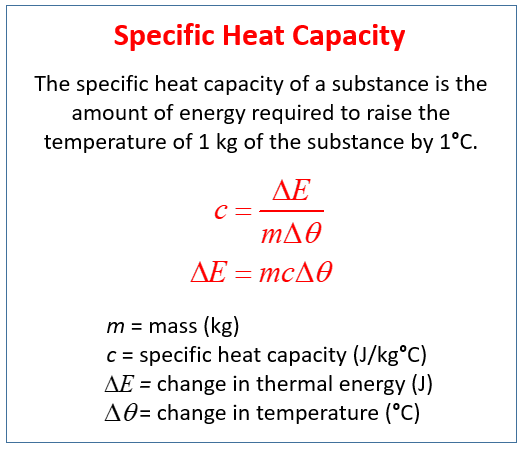
Specific Heat Capacity Video Lessons Examples Step By Step Solutions

Using Specific Heat Capacity To Find Heat Chemistry Study Com

Specific Heat Calculator Calculator Academy
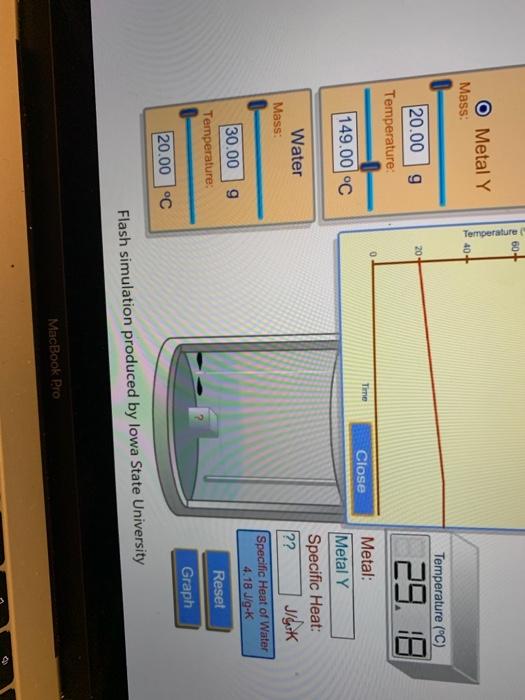
Solved List Of Metals Metal Name Specific Heat Capacity Of Chegg Com

Solved Example 12 1 Using Only P V T Data Estimate The Chegg Com
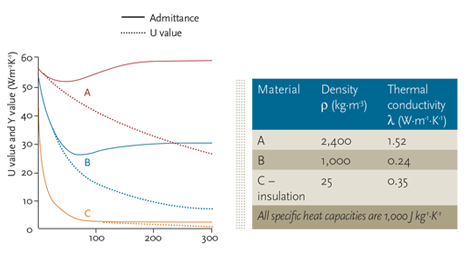
Module 48 Simple Thermal Analysis For Buildings Cibse Journal

Specific Heat Calculator

Specific Heat Capacity Problems Calculations Chemistry Tutorial Calorimetry Youtube

How To Calculate Specific Heat 6 Steps With Pictures Wikihow

How Heat Is Measured Some Specific Heat Capacity Of Substances At 25 0 C Substance Specific Heat J G 0 C Water Aluminum Copper Ppt Download